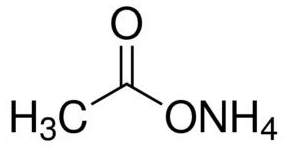
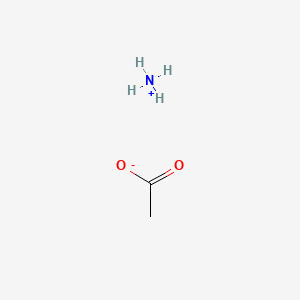
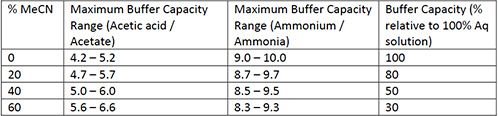
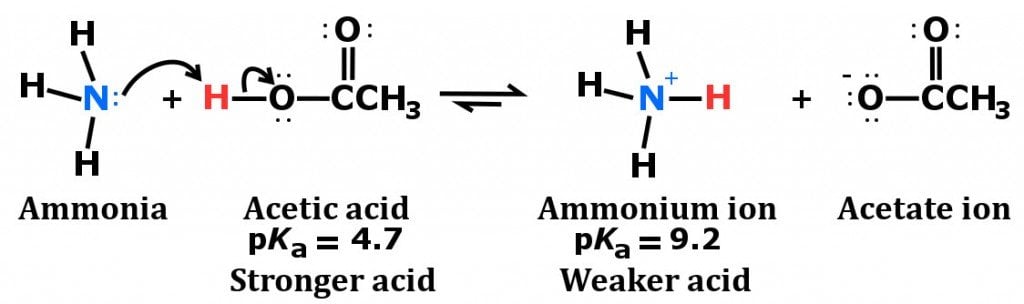
Ammonium acetate (C<sub>2</sub>H<sub>7</sub>NO<sub>2</sub>) - Structure, properties , Production, Uses and FAQs of Ammonium acetate.
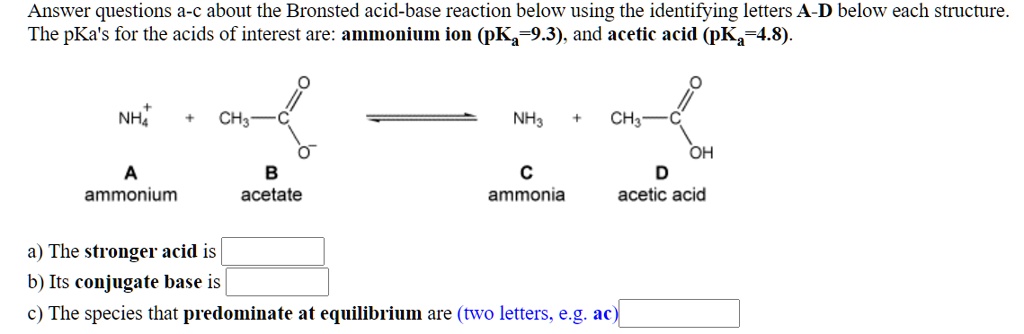
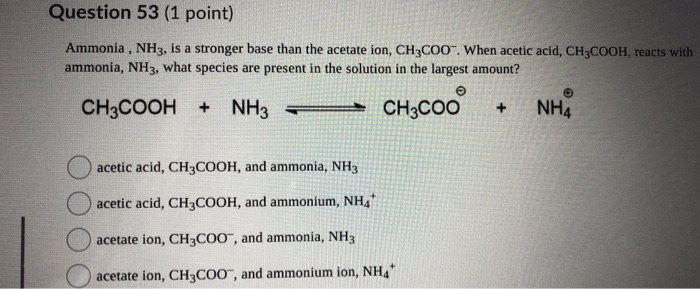
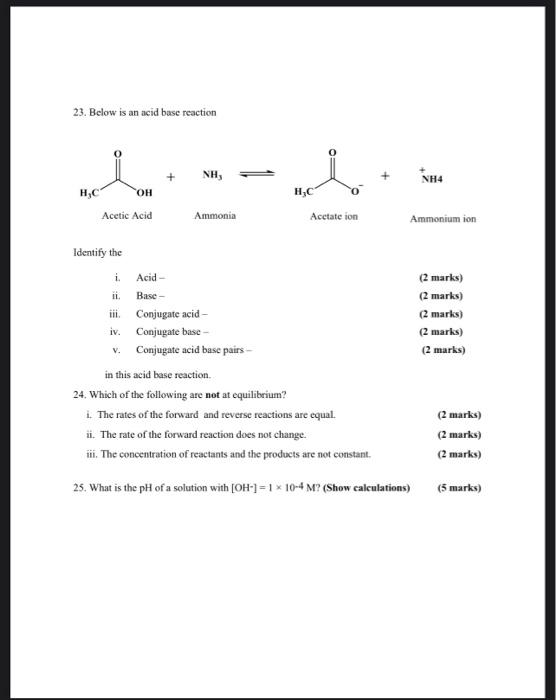
SOLVED: Answer questions a-c about the Bronsted acid-base reaction below uSing the identifying letters A-D below each structure. The pKa's for the acids of interest are: ammonium ion (pKa-9.3). and acetic acid (
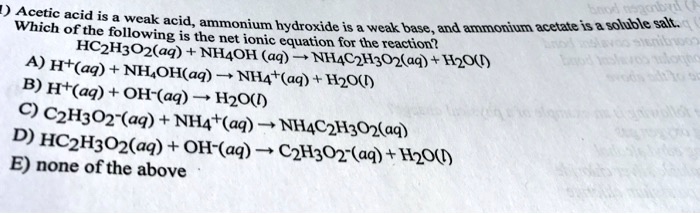
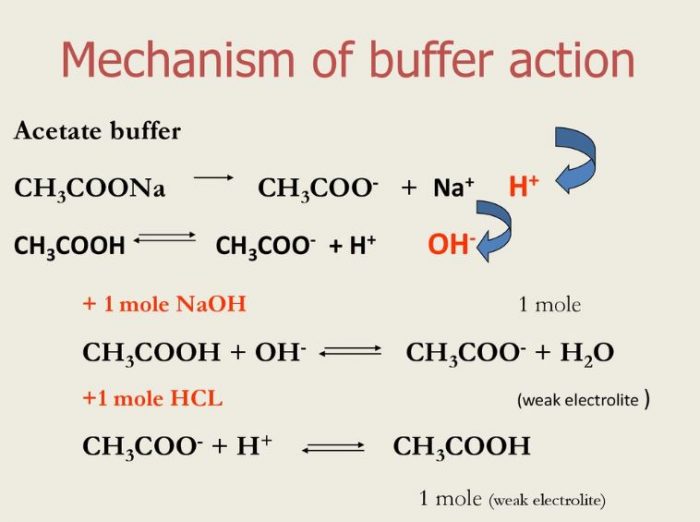
SOLVED: ) Acetic acid is Which of the wcak following acid, ammonium hydroxide is weak base , and ammonium acctate is soluble salt HC2H3Oz(aq) is the net ionic equation for the reaction?

pka of acetic acid and pKb of ammonium hydroxide are 4.76 and 4.75 respectively. Calculate the pH of ammonium acetate solution.
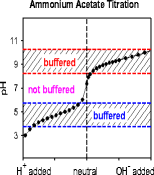
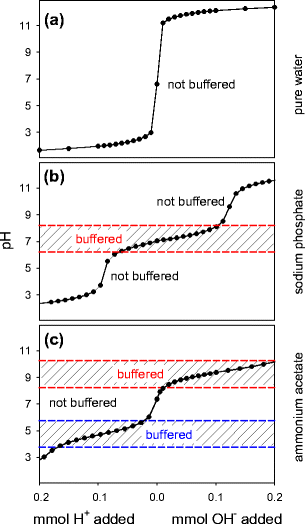
Addressing a Common Misconception: Ammonium Acetate as Neutral pH “Buffer” for Native Electrospray Mass Spectrometry | SpringerLink