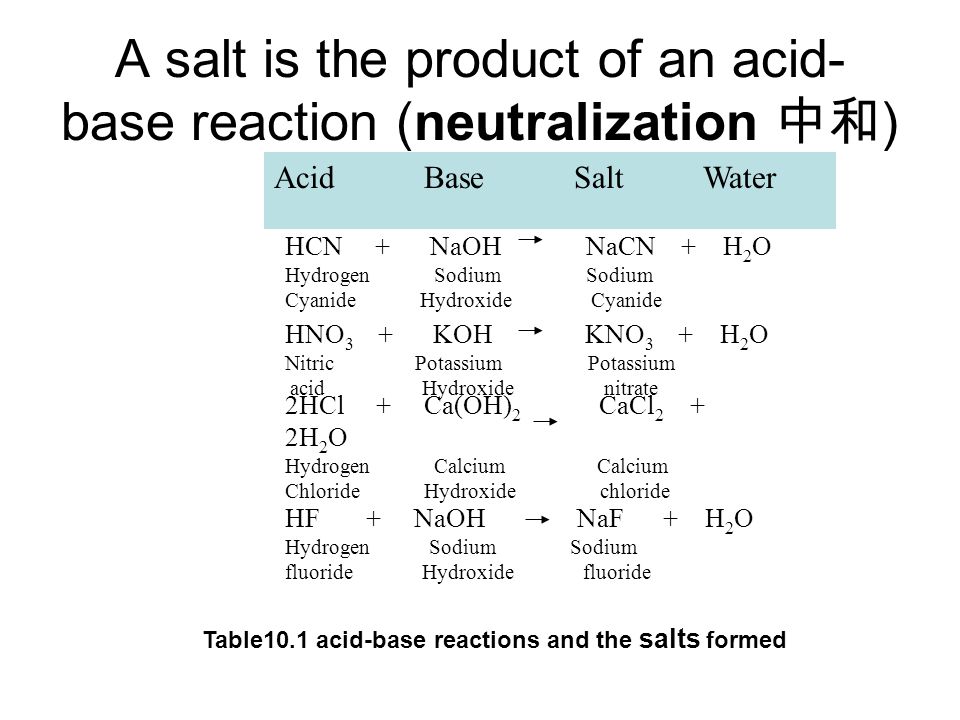
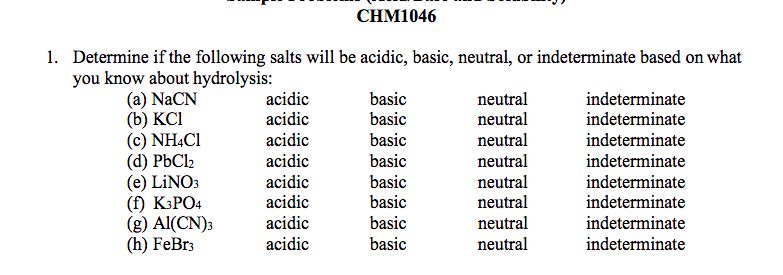
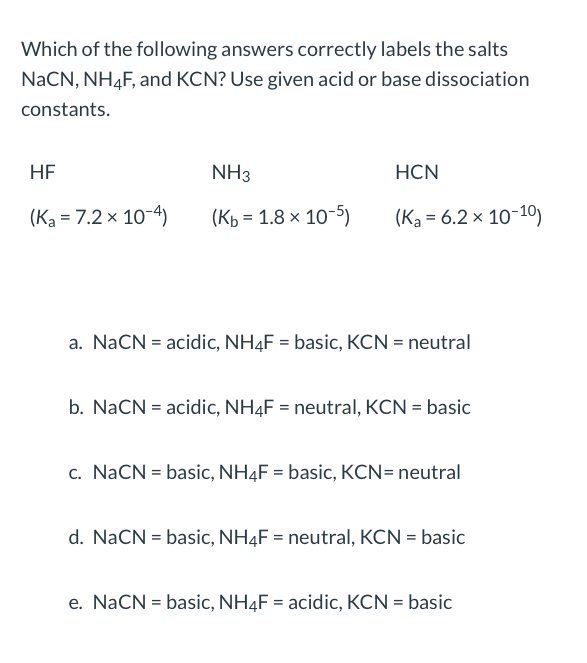
Predict if the solutions of the following salts are neutral, acidic or basic. NaCl, KBr, NaCN, NH4NO3,NaNO2 and KF

Which pair of compounds will form a buffer in aqueous solution? NaCN and KCN HCl and NaOH NaCN - Home Work Help - Learn CBSE Forum