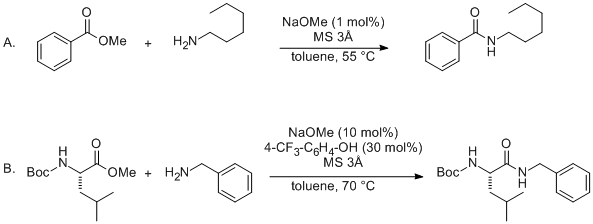
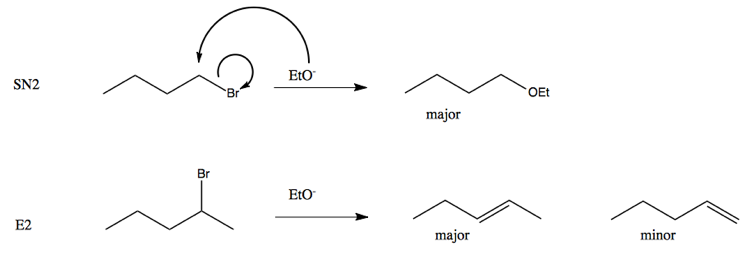
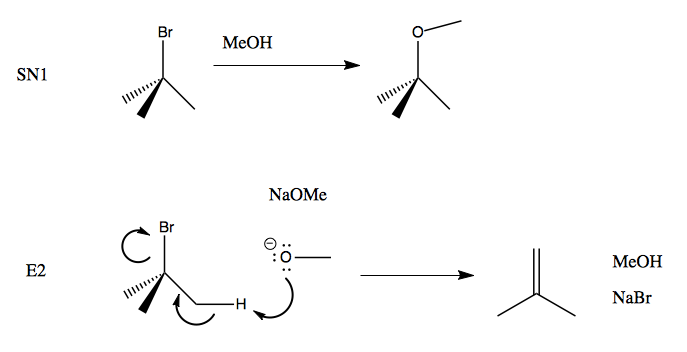
When we use a base in a reaction, why is it always preferred to use the conjugate as the solvent? For example, if NaOMe is my base, the solvent will be HOMe .

Scheme 2. Nucleophilic addition of NaOMe onto 1: acetal formation under... | Download Scientific Diagram

SOLVED: What major product results from the following E2 reaction? NaOMe MeOH Br With the small base of NaOMe, will the base remove a proton from the beta-CH2 or beta-CH when deriving

Which of the following would be the best base for performing the following elimination? A. NaOH B. NaOMe C. KOtBu D. H2O | Homework.Study.com
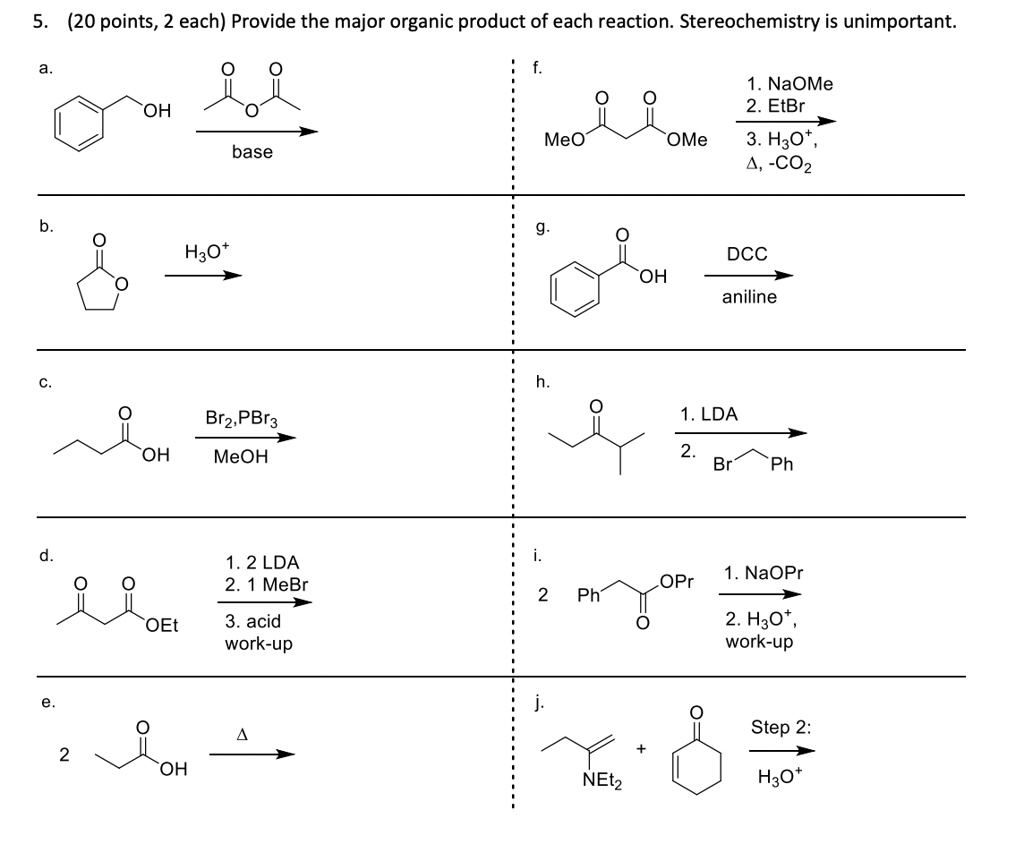
SOLVED: (20 points, 2 each) Provide the major organic product of each reaction. Stereochemistry is unimportant NaOMe 2.EtBr OH Meo OMe 3. H3O COz base Hzot DCC OH aniline 1. LDA OH

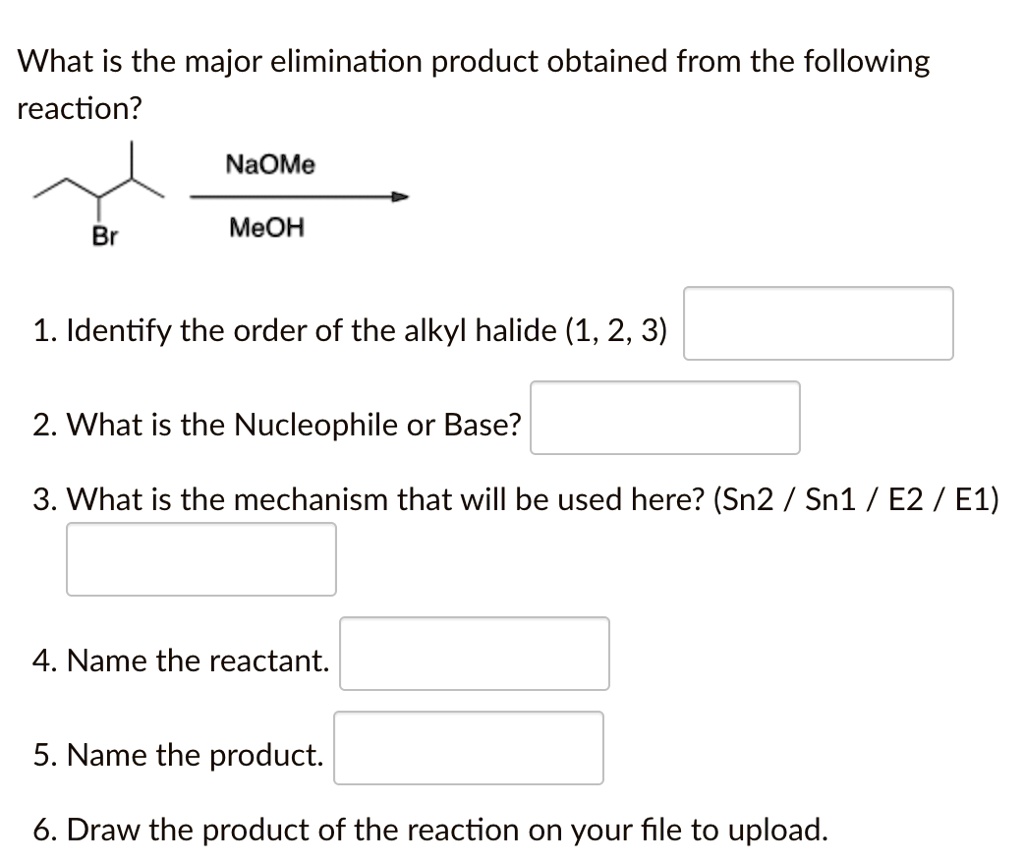
Here Meoh is used as a solvent and NaOMe is used as a nucleophile...then why is the product and elimination product rather than a substitution product?Thank u in advance community😁 : r/OrganicChemistry

Scheme 3 Reagents: (a) NaOMe, MeOH; (b) MeI, NaH, THF; (c) TsCl, Et 3... | Download Scientific Diagram
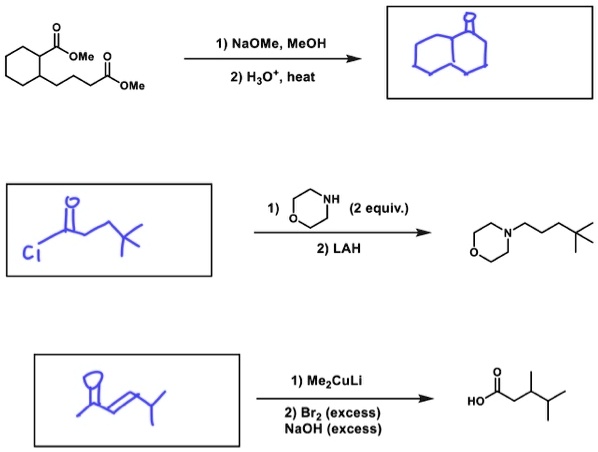
SOLVED: 1) NaOMe, MeOH OMe 2) H3O' , heat OMe (2 equiv:) 2) LAH MezCuLi Brz " (excess) NaOH (excess)

Supply structures for H through K. Given : " An Aldohexose "overset (MH2OH"/ base ")(to)H overset (Ae2O"/"NaOAC)(to)I overset (-HOAC)(to)J overset (NaOMe "/"MeOH)(to)K.