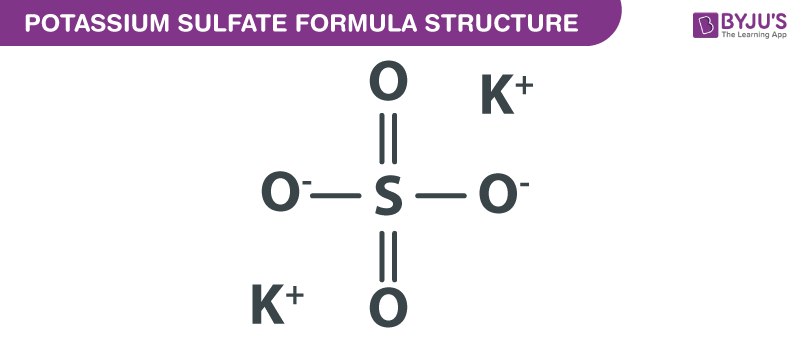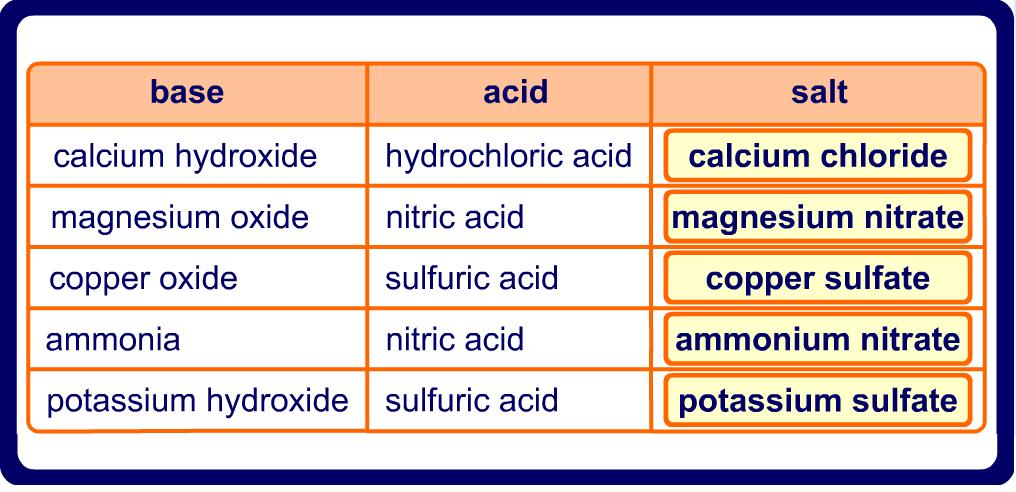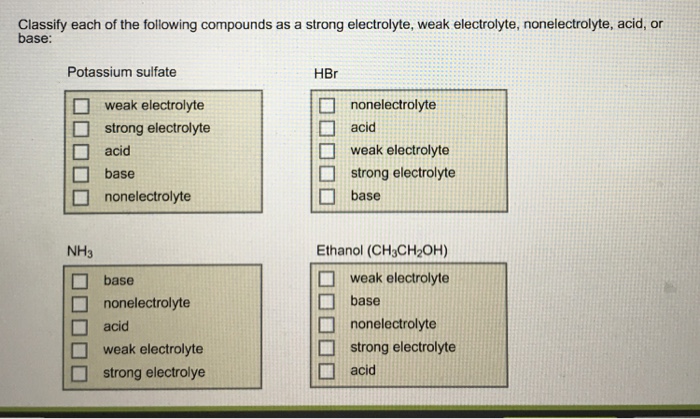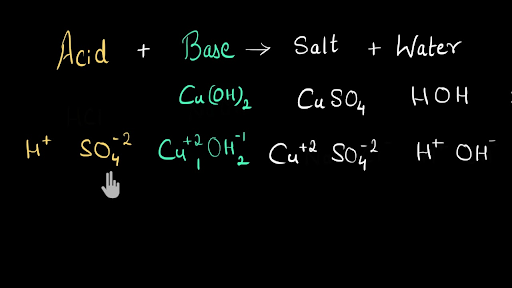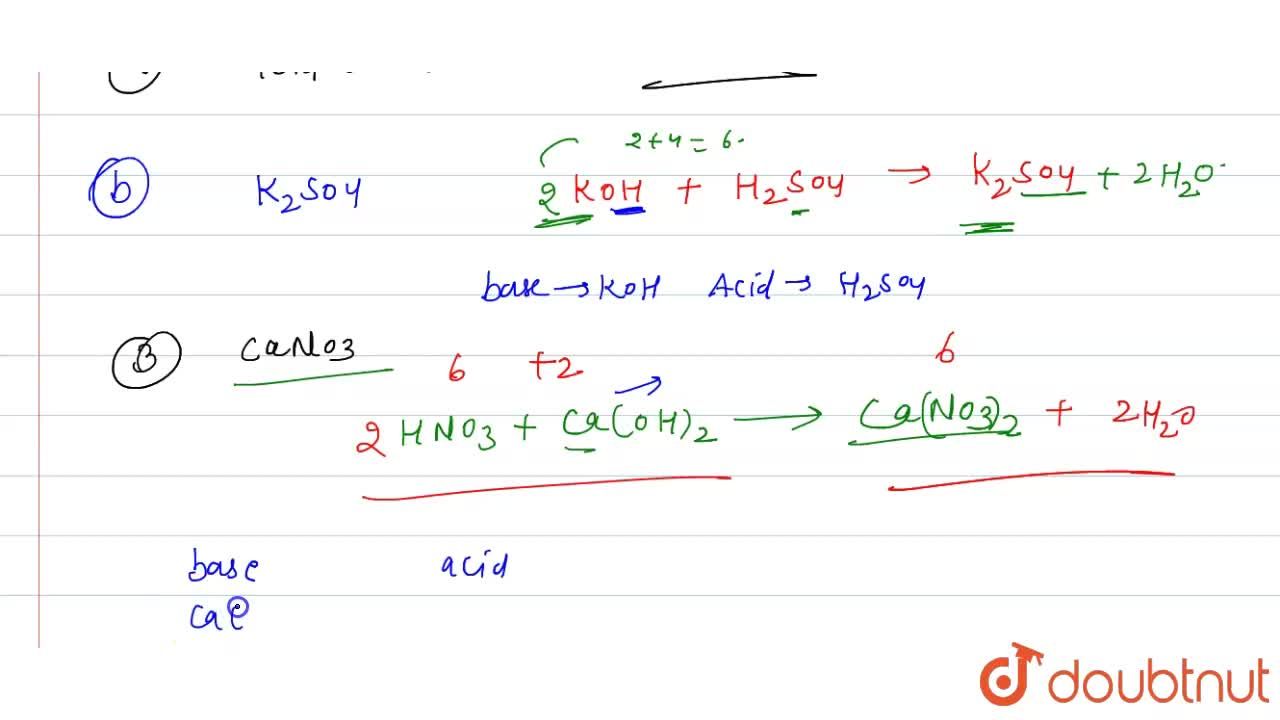
Name the acid and base from which the following salts are formed. (a) Sodium chloride (b) Potassium sulphate (c) Calcium nitrate
When sulfuric acid and potassium hydroxide neutralize each other to make water and potassium sulfate, how is water formed? | Socratic
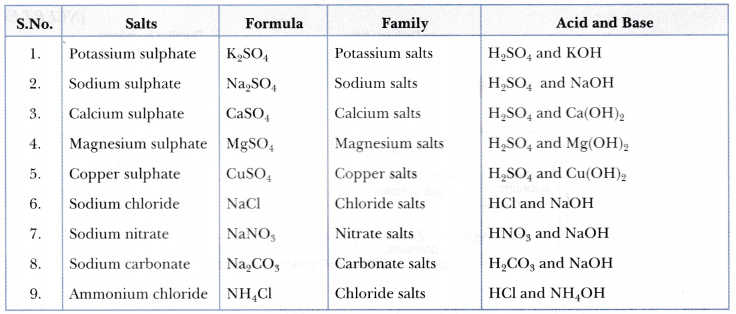
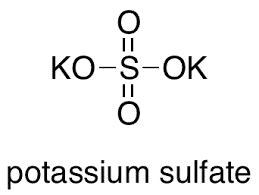
Write the formulae of the given salts as ' potassium sulphate, sodium sulphate, calcium sulphate, - CBSE Class 10 Science - Learn CBSE Forum

Write the formulae of the salts given below:Potassium sulphate, sodium sulphate, calcium sulphate, magnesium sulphate, copper sulphate, sodium chloride, sodium nitrate, sodium carbonate and ammonium chloride.Identify the acids and bases from which

How would you write the name of the following compounds ◦ Zn(OH) 2 ◦ NaOH ◦ HCl ◦ Mg(NO 3 ) 2 What does an acid do to red litmus? What does an acid to. - ppt download